 Grâce à une Recherche médico-scientifique continue, l’Implantation des Cheveux Artificiels Biocompatibles de Medicap ® 4.o notre solution moderne pour la calvitie masculine et féminine et pour les effets anti-esthétique de la perte de cheveux, s’est perfectionnée et a atteint un niveau de fiabilité et d’efficacité démontrée dans la littérature et dans les congrès plus importants à niveau international en matière de traitements anti-âge et de restauration capillaire, médecine esthétique, chirurgie plastique, esthétique et dermatologique.
Grâce à une Recherche médico-scientifique continue, l’Implantation des Cheveux Artificiels Biocompatibles de Medicap ® 4.o notre solution moderne pour la calvitie masculine et féminine et pour les effets anti-esthétique de la perte de cheveux, s’est perfectionnée et a atteint un niveau de fiabilité et d’efficacité démontrée dans la littérature et dans les congrès plus importants à niveau international en matière de traitements anti-âge et de restauration capillaire, médecine esthétique, chirurgie plastique, esthétique et dermatologique.
Les études cliniques et histologiques menées sur une période de 2, 3 et 5 ans attestent que les cheveux Artificiels Biocompatibles de Medicap ® possèdent les standards de qualité et de sécurité (haute biocompatibilité, réaction inflammatoire minime, haute résistance aux agents chimiques, physiques et mécaniques).
Une mésure additionnelle de sécurité est fournie par le particulier nœud réversible de cheveux Artificiels Biocompatibles de Medicap ® 4.0 , qui permet, au besoin, d’extraire facilement la fibre sans laisser de cicatrices.
Nous reportons ci-dessous l’avis de quelques auteurs d’études cliniques et histologiques sur l’implantation des Cheveux Artificiels Biocompatibles de Medicap® comme remède efficace pour l’alopécie masculine et féminine et comme solution aux effets anti-esthétiques de la chute de cheveux. Ces études ont été publiés dans la littérature médicale de références:
Études Cliniques
“….omissis …..we believe that hair implant will be more widespread in the future enabling to restore alopecia and scarred areas of the scalp with satisfactorily cosmetic and psychological results”. Evaluation of polyamide synthetic hair. A long-term clinical study, Palmieri B., Griselli G., D’ugo A., Palmieri G., Salti G., Panminerva Med. 2000 March; 42(1):49-53
“….omissis ….. It is a suitable methodology for surgeons, dermatologists and all those doctors who prefer a less traumatic and easier technique to satisfy those patients looking for an immediate aesthetic result….omissis ….. “. Implantation of Biocompatible Fibers for the Temporary Correction of Scalp Scars and Androgenetic Alopecia, Morselli M., Palmieri B., Santiago M., International Journal of Cosmetic Surgery and Aesthetic Dermatology 2003; 5(2):175-178
“….omissis …..These news Polyamide Fiber Implants are well tolerated by the majority of the patients and the Authors agree that this is a possible adjunctive treatment for scalp scars and a viable alternative to nonsurgical solutions (toupees, hairpieces, wigs and similar)”. Artificial Hair Fiber Restoration in the Treatment of Scalp Scars – MA Santiago, R.Perez-Rangel,A. D’Ugo, G.Griselli, G.Igitian, I.Garcia Martin, G.B.Nesheim, Usama Saad Eddin,G.smith, G.W.Brady and C.Chacker. Dermatologic Surgery 2007; 33:35-44)
“….omissis …..Artificial co-Polyamide Fibre Implantation is a safe, effective, easy and acceptable Hair Restoration Method in Androgenetic alopecia, especially in donor depleted cases” . AGRAWAL M., Modern Artificial Hair Implantation: a pilot study of 10 patients, Journal of Cosmetic Dermatology, 7(4): 315-323, July 3,2008)
Études Histologiques
“…Omissis. Our study showed the same modifications in patients without inflammatory complications as in patients with inflammatory complications, except for the presence of inflammatory infiltrates. In our cases, the density of the fibres was lower than found in patients with cutaneous complications, in particular the fibres were surrounded by closely adhering sleeves of keratin at the level of the pseudo-infundibula.
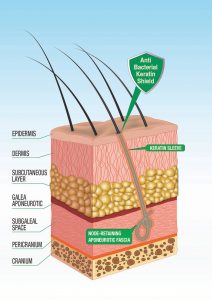 This aspect was absent in cases with inflammatory complications. In conclusion, if the hairs are implanted at low density and the physical and chemical characteristics of the fibre allow the adherence of a sleeve of keratin inside the pseudo-infundibulum, it is possible that no clinically evident inflammatory complications will appear”. Fanti P.A., Pistorale T., D’urso C., Misciali C., Tosti A., Histological study on 5 cases of patients who underwent artificial hair implantation without complications, XXXII Annual Meeting, American Society of Dermatopathology, New Orleans, USA, February 01-03, 1995
This aspect was absent in cases with inflammatory complications. In conclusion, if the hairs are implanted at low density and the physical and chemical characteristics of the fibre allow the adherence of a sleeve of keratin inside the pseudo-infundibulum, it is possible that no clinically evident inflammatory complications will appear”. Fanti P.A., Pistorale T., D’urso C., Misciali C., Tosti A., Histological study on 5 cases of patients who underwent artificial hair implantation without complications, XXXII Annual Meeting, American Society of Dermatopathology, New Orleans, USA, February 01-03, 1995
“….omissis …..Implantation of Artificial Hair (Biofibre CE 0373/TGA ) can be considered as the only alternative to treat Hair Loss for Women having severe rarefaction. The modest entity and nature of the phlogistic reaction gives credit to a high degree of biocompatibility of the fibers under examination. Rejection rate observed after pre-implant tests is acceptable (2%) and was completely solved by integrally extraction the fibers” Santiago M., Histological study of the scalp implanted with polyamide artificial fibers (Biofibre® CE 0373/TGA). Analyses after 5 years, International Society of Hair Restoration Surgery, 10th Annual Meeting, Chicago, USA, October 9-13, 2002
Pour plus d’informations sur les Produits et les Services de Medicap®





